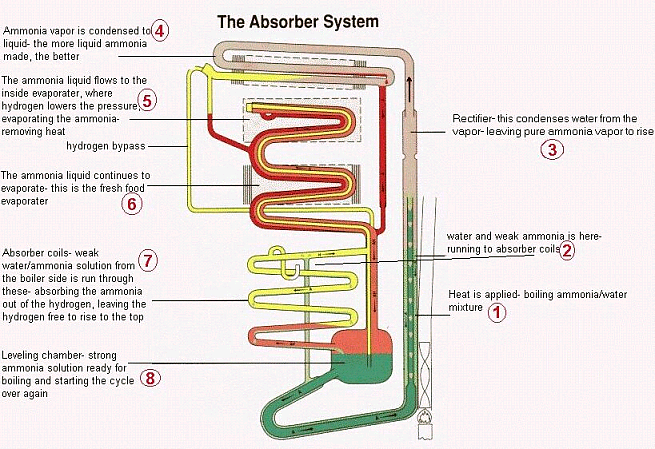
Here's a page I wrote a looong time ago explaining how an absorption unit works.
The helium has, from what I can find, a somewhat higher partial pressure than hydrogen, so the absolute lowest temp that can be achieved would be somewhat higher than with a hydrogen system. The temp for a properly charged hydrogen system is around 44 below zero, F, which is, after all, plenty cold. Not sure what the temp with helium would be.
Remember that the whole thing is due to
Dalton's Law, which states basically that the pressure of gasses in a vessel is equal to the sum of all of the pressures, but that each gas is at its own pressure, so the upper part of a cooling unit will only have hydrogen (or helium) gas present, and the pressure will be much lower than at the bottom of the unit, where the ammonia has evaporated to a gas.